Immune-Onc Programs
Blood Cancers — Immunology & Inflammation — Solid Tumors
Blood Cancer Programs
*LLS Therapy Acceleration Program (TAP) investment
**National Cancer Institute (NCI) and California Institute of Regenerative Medicine (CIRM) Grants
-
LILRB4, also known as ILT3, is an immune-modulatory transmembrane protein found on monocytes and monocyte-derived cells. LILRB4 is expressed on certain hematologic cancer cells, such as monocytic and myelomonocytic leukemia blasts.
-
IO-202 is a first-in-class IgG1 antibody with specific, high-affinity binding to LILRB4 and depletes LILRB4 positive cells via antibody-dependent cellular cytotoxicity and antibody-dependent cellular phagocytosis. As such, IO-202 is a targeted therapy with broad potential in blood cancers.
IO-202 has completed the dose escalation part of the first-in-human, multicenter, open-label Phase 1 study in the U.S., and the data was presented at the European Hematology Association (EHA) Congress in 2023. This Phase 1 trial has advanced to the dose expansion stage to evaluate IO-202 in combination with azacitidine (NCT04372433) in patients with newly diagnosed chronic myelomonocytic leukemia (CMML) who have not received any hypomethylating agents (HMA). Data from the Phase 1b CMML expansion cohort were highlighted in an oral presentation at the 2024 American Society of Hematology (ASH) Annual Meeting in San Diego, California.
The U.S. Food and Drug Administration granted IO-202 Fast Track Designations for the treatment of patients with relapsed or refractory acute myeloid leukemia (AML) and relapsed or refractory CMML, respectively. The FDA has also granted IO-202 Orphan Drug Designations for the treatment of AML and CMML, respectively.
-
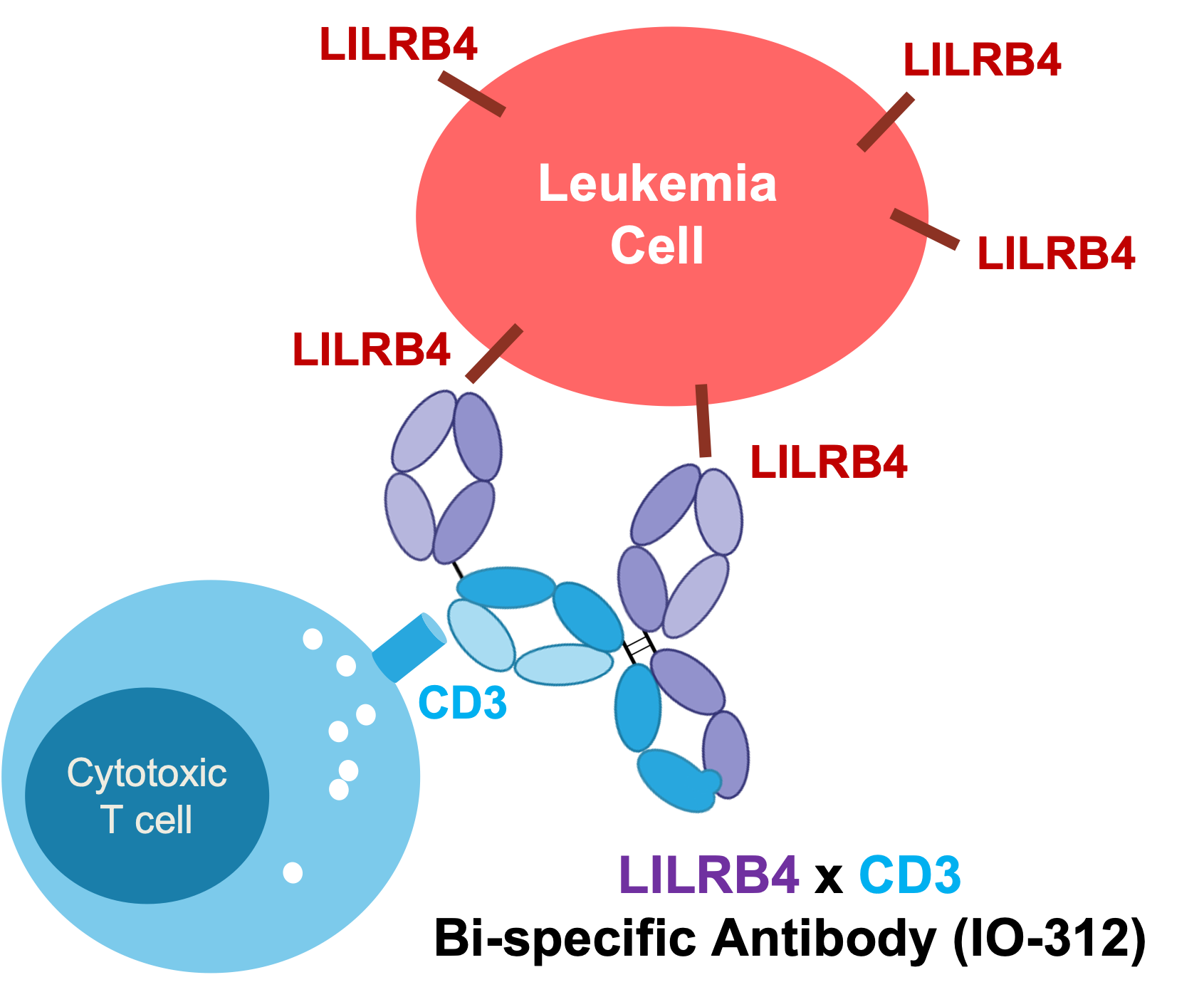
Engineered and optimized using a proprietary bispecific antibody platform, IO-312 is a first-in-class LILRB4-directed bispecific monoclonal antibody with potential against AML and other hematologic malignancies. Preclinical data presented at the 2023 American Association for Cancer Research Annual Meeting demonstrate that IO-312 led to potent and specific killing of monocytic AML cells in vitro and in vivo. Additional design features include high affinity and specific binding to LILRB4 as well as low affinity binding to CD3 to maximize anti-tumor effect without cytokine release-related toxicities, excellent biophysical properties for efficient manufacturing, and cross-reactivity to non-human primate LILRB4 and CD3 to enable preclinical toxicology assessment for rapid advancement to the clinic.